
Screening Criteria
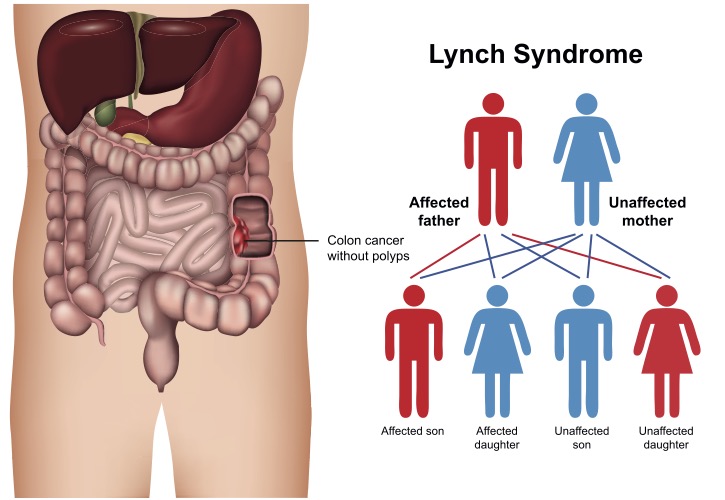
Screening for Lynch syndrome has evolved over time. The primary factors for these changes is knowledge and cost of genetic testing. Current guidelines support screening everybody with a personal history of colorectal or endometrial (uterine) cancers. If a family member has one of these 2 cancers ideally this affected family member should be tested for Lynch syndrome. If a family member has Lynch syndrome cascade testing or testing of all members up and down the family tree should be undertaken. Since Lynch syndrome is autosomal dominant there is a 50% chance of inheriting it from an affected parent. A detailed family history of all cancers is important to help determine the risk of Lynch syndrome. This is crucial since Lynch syndrome can cause cancers in most organ systems. These include colorectal, endometrial, gastric, ovarian, pancreatic, ureter and renal pelvis, biliary tract, brain (glioblastoma), and small intestinal cancers. Dermatologic manifestations include sebaceous adenomas, sebaceous carcinomas, and keratoacanthomas
The history of "Lynch syndrome" is fascinating. Dr. Henry Lynch dedicated his life to studying families who had multiple cancers. He was perplexed because some members of these “Cancer Families” had colon cancer in an autosomal dominant pattern but did not have multiple polyps indicative of familial adenomatous polyposis (FAP). Further research noted that these families also had a significant increase in other cancers - especially those of the uterus and ovaries.
This hereditary non-polyposis colorectal cancer (HNPCC) syndrome was perplexing. He joined other leading experts in creating the Amsterdam and Bethesda Guidelines for identification of HNPCC. Since many individuals with HNPCC will never get colorectal cancer, changing the name to Lynch syndrome avoids confusion and honors Dr. Henry Lynch's work.
PREMM5 Model - Online Lynch Syndrome Risk Calculator
Amsterdam Criteria
An international collaborative group met in Amsterdam in 1991 to establish guidelines for identifying Lynch Syndrome families.
- Three affected relatives with colorectal cancer, one of them a first-degree relative of the other two.
- AND colorectal cancer in at least 2 generations.
- One family member diagnosed with colorectal cancer before age 50 years.
- AND Familial Adenomatous Polyposis (FAP) should be excluded.
Amsterdam Criteria II
Because genetically abnormal families were identified that did not meet the above guidelines, a revised criteria were published in 1999.
- There should be at least three relatives with a Lynch Syndrome –associated cancer (colorectal, endometrial, small bowel, ureter or renal pelvis).
- One should be a first-degree relative of the other two.
- At least two successive generations should be affected.
- At least one should be diagnosed before age 50 years.
- Familial adenomatous polyposis should be excluded in the colorectal cancer cases.
Revised Bethesda Guidelines
A third set of criteria can also be used to identify Lynch Syndrome families.
- Colorectal cancer diagnosed in an individual younger then 50 years.
- Presence of synchronous metachronous colorectal, or other Lynch Syndrome–associated tumors in an individual regardless of age.
- Colorectal cancer with MSI-high pathologic associated features diagnosed in an individual younger than 60 years.
- Colorectal cancer or Lynch Syndrome-associated tumor diagnosed in at least one first –degree relative younger than 50 years.
- Colorectal cancer or Lynch Syndrome-associated tumor diagnosed at any age in two first-degree or second-degree relatives.
3-2-1 rule
- 3 relatives with colorectal cancer &
- 2 successive generations involved &
- 1 person diagnosed before age 50.
(familial adenomatous polyposis should be excluded)

